
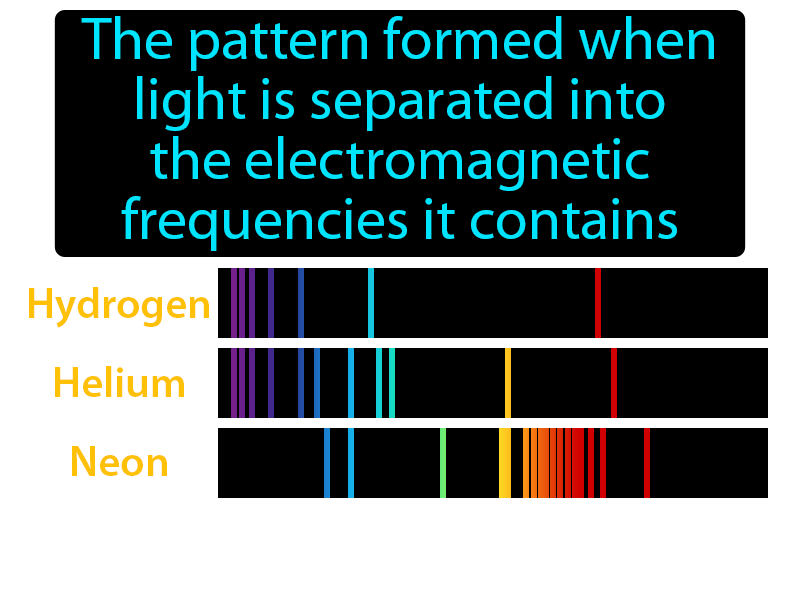
Several of the possible emissions are observed because the sample contains many hydrogen atoms that are in different initial energy states and reach different final energy states. If only a single atom of hydrogen were present, then only a single wavelength would be observed at a given instant. The above picture shows the visible light emission spectrum for hydrogen. When the electron falls back to its ground level the light is emitted. When excited, an electron moves to a higher energy level or orbital. The frequencies of light that an atom can emit are dependent on states the electrons can be in. The principle of the atomic emission spectrum explains the varied colors in neon signs, as well as chemical flame test results (described below). This concludes that only photons with specific energies are emitted by the atom. Each of these frequencies are related to energy by the formula:Į photon = h ν, is Planck's constant. The fact that only certain colors appear in an element's atomic emission spectrum means that only certain frequencies of light are emitted.

These emitted photons form the element's spectrum. The wavelength (or equivalently, frequency) of the photon is determined by the difference in energy between the two states. When the electrons fall back down and leave the excited state, energy is re-emitted in the form of a photon. When the electrons in the atom are excited, for example by being heated, the additional energy pushes the electrons to higher energy orbitals. The description has been superseded by quantum electrodynamics, although the semi-classical version continues to be more useful in most practical computations. The quantum mechanics problem is treated using time-dependent perturbation theory and leads to the general result known as Fermi's golden rule. Precise measurements at many wavelengths allow the identification of a substance via emission spectroscopy.Įmission of radiation is typically described using semi-classical quantum mechanics: the particle's energy levels and spacings are determined from quantum mechanics, and light is treated as an oscillating electric field that can drive a transition if it is in resonance with the system's natural frequency. This may be related to other properties of the object through the Stefan–Boltzmann law.įor most substances, the amount of emission varies with the temperature and the spectroscopic composition of the object, leading to the appearance of color temperature and emission lines. The emittance of an object quantifies how much light is emitted by it. On the other hand, nuclear shell transitions can emit high energy gamma rays, while nuclear spin transitions emit low energy radio waves. For example, visible light is emitted by the coupling of electronic states in atoms and molecules (then the phenomenon is called fluorescence or phosphorescence). The energy states of the transitions can lead to emissions over a very large range of frequencies. Since energy must be conserved, the energy difference between the two states equals the energy carried off by the photon. The frequency of light emitted is a function of the energy of the transition. In physics, emission is the process by which a higher energy quantum mechanical state of a particle becomes converted to a lower one through the emission of a photon, resulting in the production of light. Similarly, the emission spectra of molecules can be used in chemical analysis of substances. Therefore, spectroscopy can be used to identify elements in matter of unknown composition. Each element's emission spectrum is unique. This collection of different transitions, leading to different radiated wavelengths, make up an emission spectrum. There are many possible electron transitions for each atom, and each transition has a specific energy difference. The photon energy of the emitted photons is equal to the energy difference between the two states. The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to electrons making a transition from a high energy state to a lower energy state. A demonstration of the 589 nm D 2 (left) and 590 nm D 1 (right) emission sodium D lines using a wick with salt water in a flame

Frequencies of light emitted by atoms or chemical compounds Emission spectrum of a ceramic metal halide lamp.


 0 kommentar(er)
0 kommentar(er)
